The development of rapid, highly sensitive, and versatile detection method for viral pathogens is of critical importance in clinical diagnosis, food safety, and biodefense [1]. Virus isolation in tissue culture, nucleic acid probe-based methods (PCR, LCR), and immunological approaches are the most common techniques available in laboratories for virus detection and identification [2]. The culturing method is usually laborious and time consuming, that may require several weeks to perform. The utility of PCR assays which employ amplification is generally limited by the occurrence of false-positive results and the requirement of trained operators or expensive equipment. Enzyme-linked immunosorbent assay (ELISA) combines the specificity of antibodies with high catalytic activity of enzymes to provide highly selective, quantitative and reproducible results on the mirowell plate-based platform. However, limited by the low sensitivity, most conventional ELISA systems fail to monitor the pathogens at an early infection stage when their concentrations are generally very low. In addition, conventional ELISA usually require large volumes in each assay and take a long time for incubation and blocking steps [3].
Magnetic particles, which are generally considered to be biologically and chemically inert, have been widely used as a powerful tool for a wide range of biomedical and bioanalytical applications [4]. On one hand, magnetic nanoparticles (MNPs) have been demonstrated to possess an intrinsic enzyme mimetic activity similar to that of the natural peroxidases, which can be further used to construct highly sensitive and selective sensors for metal ions [5], glucose [6], proteins [7], and DNA [8] through their catalytic oxidation of various substrates. On the other hand, the superparamagnetic property of magenetic beads (MBs), which can be magnetically controlled with an external magnetic field and immediately redispersed with the removing of magnet, offer a great potential for numerous biomedical applications, such as cell separation [9], automated DNA/RNA extraction [10], gene targeting [11], and drug delivery [12]. Moreover, when coated with, for example, an antibody, they can act as ideal solid supports in immunoassays because of their biocompatibility, stability, and easy surface modification, allowing a straightforward method to capture target analytes of interest from large volume samples without pre-enrichment or purification steps. Meanwhile, MBs possess large surface-to-volume ratio that can provide a comparably large numbers of binding sites for immunoreactions and offer higher interaction efficiency between the samples and reagents, resulting in faster assay kinetics and a lower detection limit [13]. In addition, immunoassay using MBs as the separation tool enable the detection to proceed in the aqueous solution, eliminating multiple washing steps and reducing the consumption of samples and reagents, which in turn minimizes the analysis cost, time and complexity. Taken together, MBs have emerged as a unique alternative substrate for the fabrication of novel immunoassays.
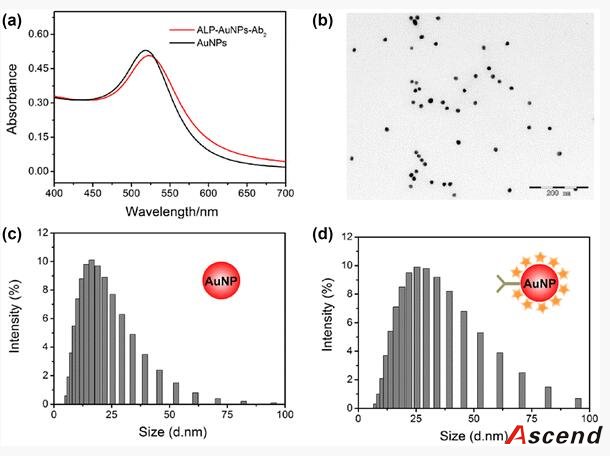
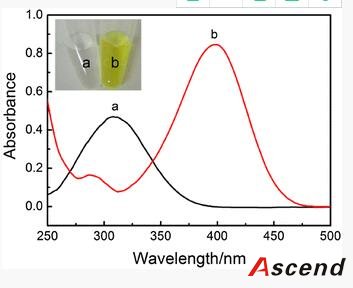
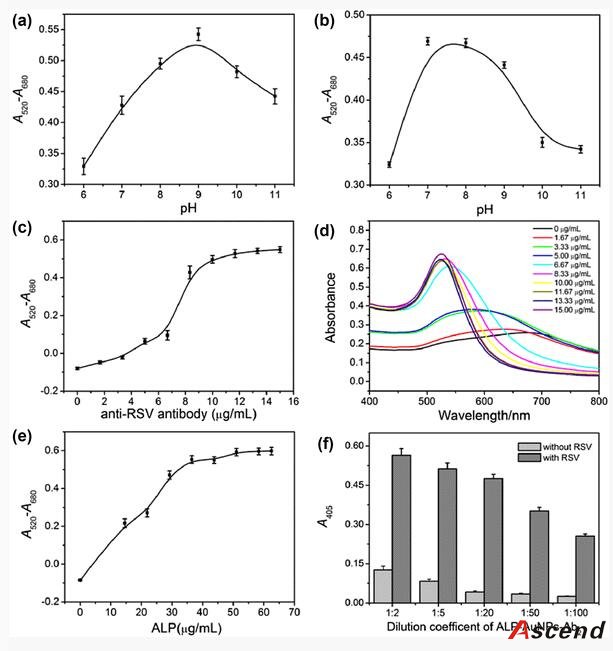
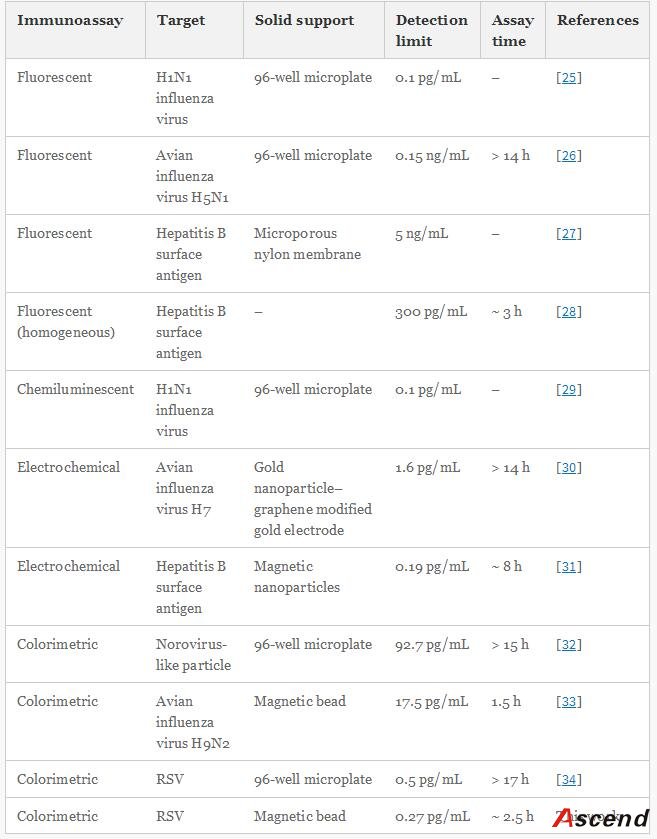
Furthermore, to improve the detection sensitivity of ELISA, incorporation of an additional signal amplification strategy is necessary. Up until now, some successful signal amplification systems utilizing enzymatic biocatalytic precipitation techniques or nanomaterials including liposomes and nanoparticles to load a large amount of tags have been proposed [14, 15, 16, 17]. Among these strategies, the nanoparticle-based amplification has received a great deal of interest and caused a significant impact in immunoassay. In particular, gold nanoparticles (AuNPs) are very attractive materials for signal amplification in immunoassay due to their unique properties, such as good biological compatibility, high loading capacity, and stability [18]. Ambrosi et al. have fabricated an enhanced ELISA for the analysis of CA15–3 antigen using AuNPs as carriers of the signaling antibody anti-CA15–3-HRP (horseradish peroxidase) [19]. Though the assay holds higher sensitivity and shorter assay time when compared to conventional ELISA procedures, it suffers from complicated and expensive conjugation process of preparing HRP-linked antibodies. Lin et al. further increased HRP loading amount and thus reduced the detection limit and cost greatly by employing a combination of AuNPs and graphene oxide sheets as carriers [20]. However, this approach often requires multiple labels and multicycle operations for introducing nanoconjugates, resulting in the complicated, laborious, and time-consuming procedure.
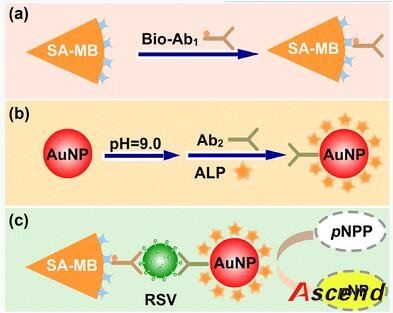
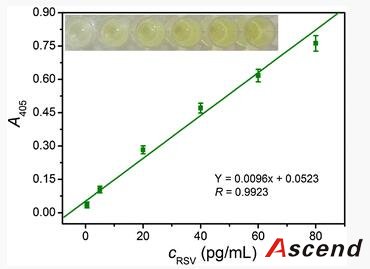
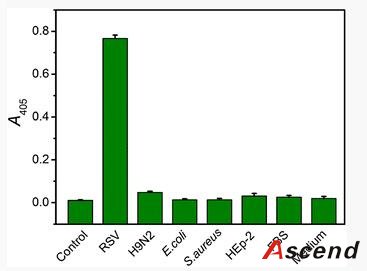
The work herein employs MBs as solid support and AuNPs as carrier to construct an enhanced immunoassay for the rapid, low-level detection of respiratory syncytial virus (RSV), which acts as a model target of pathogenic viruses. RSV, a single-stranded RNA virus of the paramyxocirus family, not only mediates serious lower respiratory tract illness in infants and toddlers which causes repeat infections throughout life, but also acts as a significant pathogen of the elderly and immune compromised patients [21]. Therefore, rapid, specific, and sensitive diagnosis of RSV is important to public-health monitoring.